17.10.2006
Though in clinical use for decades, a small, sweet-tasting compound is revealing a startling new face as a potential cure for epilepsy.
2-deoxy-glucose, or 2DG, has long been used in radio labeling, medical scanning and cancer imaging studies in humans. But now, researchers at the University of Wisconsin-Madison have found the substance also blocks the onset of epileptic seizures in laboratory rats.
Reported in the journal Nature Neuroscience, the findings have potentially huge implications for up to half of all epileptic patients who currently have no access to treatment, says senior author Avtar Roopra, a UW-Madison assistant professor of neurology."We pumped the rats full [of 2DG] and still saw no side effects," says Roopra, who estimates that the compound may be available for human use within five years. "I see 2DG as an epilepsy management treatment much like insulin is used to treat diabetes." "All the available epilepsy treatments have focused on suppressing seizures," says co-author and renowned epilepsy expert Tom Sutula, a UW-Madison professor of neurology. "There has been hope that [new drugs] will not only suppress seizures, but modify their consequences. [2DG] appears to be a novel treatment that offers great promise to achieve that vision." About 1 percent of the world's population suffers from epilepsy, a neurological condition that makes people susceptible to seizures. Scientists believe that seizures, of which there are many kinds, occur due to sudden changes in how brain cells send electrical signals to each other. In about 30 to 50 percent of epilepsy patients, available treatments - including the removal of parts of the brain's temporal lobe - are largely ineffective.2DG is essentially a more palatable version of the "ketogenic," or sugar-free, diets that some researchers have long recommended to epilepsy patients. Indeed, the notion of a sugar-free diet actually stretches back thousands of years to Biblical times, when healers sometimes prescribed starvation as a potent way to fend off seizures. UW-Madison researchers first began to investigate the role of sugar in controlling seizures after early experiments showed that children on sugar-free diets can rapidly experience seizures when they consume even a small dose of carbohydrates, such as a cookie or a little piece of bread.But ketogenic regimens can be a miserable experience. "The kids can't eat any sugar at all. Imagine no bread or Christmas cake," says Roopra. But 2DG would work as an effective substitute because it enters cells and clogs up certain cellular enzymes. As a result, the body can't use its own glucose.Though ketogenic diets seem to work in many epilepsy patients in whom existing treatments have been unsuccessful, scientists have struggled to understand the exact cellular connection between no sugar and no seizures. The UW-Madison work for the first time clears up some of that mystery. Roopra has long explored how certain proteins known as "transcription factors" turn neuronal genes on or off. He has been particularly intrigued by one transcription factor known as NRSF, which is thought to control up to 1,800 genes in the brain, including many that are implicated in epilepsy. Like an electrical motherboard, NRSF ensures that neuronal genes switch "on" in the body's neurons, while remaining switched "off" in other regions where they normally play no role. Roopra found that NRSF binds to another protein called CTBP. The finding "immediately raised alarm bells," Roopra says, because CTBP also binds to a free-floating molecule - NADH - that emerges when sugars break down in cells. To his surprise, Roopra found that CTBP binds to either NRSF or NADH. In other words, a cell with a lot of glucose generates a lot of NADH, so CTBP is more likely to bind with the sugar byproduct than NRSF. But without CTBP, NRSF most likely derails the normal function of certain neuronal genes - including those connected to epilepsy.Scientists believe that NSRF also controls genes that potentially play a role in cancer. Roopra is planning future studies to test whether 2DG holds promise for combating breast cancer, or fast-spreading glioblastomas. The UW-Madison team has patented 2DG for its use against epilepsy in collaboration with the Wisconsin Alumni Research Foundation, UW-Madison's technology transfer arm.
Tuesday, October 31, 2006
Friday, October 13, 2006
MEMORY MATTERS
First of all, We have to know what is Memory?
When an event happens, when you learn something, or when you meet someone, your brain determines whether that information needs to be saved. If your brain judges the information important, it places it in your memory as like "files." You probably know your brain has different analitical parts. Some of them are important for memory. The hippocampus (say: hih-puh-kam-pus) is one of the more important parts of the brain that processes memories. Old information and new information, or memories, are thought to be processed and stored away in different areas of the cerebral cortex, or the "gray matter" of the brain - the largest, outermost part of the brain.
Let us know more about hippocampus.
The hippocampus is a part of the brain located inside the temporal lobe (humans and other mammals have two hippocampi, one in each side of the brain). It forms a part of the limbic system and plays a part in memory and spatial navigation. The name derives from its curved shape in coronal sections of the brain, which resembles a seahorse (Greek: hippo=horse, kampos=sea monster). In Alzheimer's disease, the hippocampus becomes one of the first regions of the brain to suffer damage; memory problems and disorientation appear amongst the first symptoms. Damage to the hippocampus can also result from oxygen starvation (anoxia) and encephalitis. In the anatomy of animals, the hippocampus is among the phylogenetically oldest parts of the brain. The hippocampal emergence from the archipallium is most pronounced in primates and Cetacean sea mammals. Nonetheless, in primates the hippocampus occupies less of the cerebrum in proportion to cerebral cortex among the youngest species, especially humans. The significant development of hippocampal volume in primates correlates more with overall increase of brain mass than with neocortical development.
ANATOMY OF HIPPOCAMPUS
Although there is a lack of consensus relating to terms describing the hippocampus and the adjacent cortex, the term hippocampal formation generally applies to the dentate gyrus, the Cornu Ammonis fields CA1-CA3 (and CA4, frequently called the hilus and considered part of the dentate gyrus), and the subiculum. The CA1, CA2 and CA3 fields make up the hippocampus proper.
Information flow through the hippocampus proceeds from dentate gyrus to CA3 to CA1 to the subiculum, with additional input information at each stage and outputs at each of the two final stages. CA2 represents only a very small portion of the hippocampus and its presence is often ignored in accounts of hippocampal function, though it is notable that this small region seems unusually resistant to conditions that usually cause large amounts of cellular damage, such as epilepsy.
The perforant path, which brings information primarily from entorhinal cortex (but also perirhinal cortex, among others), is generally considered the main source of input to the hippocampus. Layer II of entorhinal cortex (EC) brings input to the dentate gyrus and field CA3, while EC layer III brings input to field CA1 and the subiculum. The main output pathways of the hippocampus are the cingulum bundle and the fimbria/fornix, which arise from field CA1 and the subiculum.

Diagram of hippocampus
Perforant path input from EC layer II enters the dentate gyrus and is relayed to region CA3 (and to mossy cells, located in the hilus of the dentate gyrus, which then send information to distant portions of the dentate gyrus where the cycle is repeated). Region CA3 combines this input with signals from EC layer II and sends extensive connections within the region and also sends connections to region CA1 through a set of fibers called the Schaffer collaterals. Region CA1 receives input from the CA3 subfield, EC layer III and the nucleus reuniens of the thalamus (which project only to the terminal apical dendritic tufts in the stratum lacunosum-moleculare). In turn, CA1 projects to the subiculum as well as sending information along the aforementioned output paths of the hippocampus. The subiculum is the final stage in the pathway, combining information from the CA1 projection and EC layer III to also send information along the output pathways of the hippocampus.
The hippocampus also receives a number of subcortical inputs. In Macaca fascicularis, these inputs include the amygdala (specifically the anterior amygdaloid area, the basolateral nucleus, and the periamygdaloid cortex), the medial septum and the diagonal band of Broca, the claustrum, the substantia innominata and the basal nucleus of Meynert, the thalamus (including the anterior nuclear complex, the laterodorsal nucleus, the paraventricular and parataenial nuclei, the nucleus reuniens, and the nucleus centralis medialis), the lateral preoptic and lateral hypothalamic areas, the supramammillary and retromammillary regions, the ventral tegmental area, the tegmental reticular fields, the raphe nuclei (the nucleus centralis superior and the dorsal raphe nucleus), the nucleus reticularis tegementi pontis, the central gray, the dorsal tegmental nucleus, and the locus coeruleus.
It is widely accepted that each of these regions has a unique functional role in the information processing of the hippocampus, but to date the specific contribution of each region is poorly understood.
Information flow through the hippocampus proceeds from dentate gyrus to CA3 to CA1 to the subiculum, with additional input information at each stage and outputs at each of the two final stages. CA2 represents only a very small portion of the hippocampus and its presence is often ignored in accounts of hippocampal function, though it is notable that this small region seems unusually resistant to conditions that usually cause large amounts of cellular damage, such as epilepsy.
The perforant path, which brings information primarily from entorhinal cortex (but also perirhinal cortex, among others), is generally considered the main source of input to the hippocampus. Layer II of entorhinal cortex (EC) brings input to the dentate gyrus and field CA3, while EC layer III brings input to field CA1 and the subiculum. The main output pathways of the hippocampus are the cingulum bundle and the fimbria/fornix, which arise from field CA1 and the subiculum.

Diagram of hippocampus
Perforant path input from EC layer II enters the dentate gyrus and is relayed to region CA3 (and to mossy cells, located in the hilus of the dentate gyrus, which then send information to distant portions of the dentate gyrus where the cycle is repeated). Region CA3 combines this input with signals from EC layer II and sends extensive connections within the region and also sends connections to region CA1 through a set of fibers called the Schaffer collaterals. Region CA1 receives input from the CA3 subfield, EC layer III and the nucleus reuniens of the thalamus (which project only to the terminal apical dendritic tufts in the stratum lacunosum-moleculare). In turn, CA1 projects to the subiculum as well as sending information along the aforementioned output paths of the hippocampus. The subiculum is the final stage in the pathway, combining information from the CA1 projection and EC layer III to also send information along the output pathways of the hippocampus.
The hippocampus also receives a number of subcortical inputs. In Macaca fascicularis, these inputs include the amygdala (specifically the anterior amygdaloid area, the basolateral nucleus, and the periamygdaloid cortex), the medial septum and the diagonal band of Broca, the claustrum, the substantia innominata and the basal nucleus of Meynert, the thalamus (including the anterior nuclear complex, the laterodorsal nucleus, the paraventricular and parataenial nuclei, the nucleus reuniens, and the nucleus centralis medialis), the lateral preoptic and lateral hypothalamic areas, the supramammillary and retromammillary regions, the ventral tegmental area, the tegmental reticular fields, the raphe nuclei (the nucleus centralis superior and the dorsal raphe nucleus), the nucleus reticularis tegementi pontis, the central gray, the dorsal tegmental nucleus, and the locus coeruleus.
It is widely accepted that each of these regions has a unique functional role in the information processing of the hippocampus, but to date the specific contribution of each region is poorly understood.
Role of Hippocampus in General Memory
Drawing of the neural circuitry of the rodent hippocampus. S. Ramón y Cajal, 1911.Psychologists and neuroscientists dispute the precise role of the hippocampus, but, in general, agree that it has an essential role in the formation of new memories about experienced events (episodic or autobiographical memory). Some researchers prefer to consider the hippocampus as part of a larger medial temporal lobe memory system responsible for general declarative memory (memories that can be explicitly verbalized — these would include, for example, memory for facts in addition to episodic memory). Some evidence supports the idea that, although these 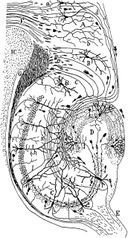 forms of memory often last a lifetime, the hippocampus ceases to play a crucial role in the retention of the memory after a period of consolidation. Damage to the hippocampus usually results in profound difficulties in forming new memories (anterograde amnesia), and normally also affects access to memories prior to the damage (retrograde amnesia). Although the retrograde effect normally extends some years prior to the brain damage, in some cases older memories remain - this sparing of older memories leads to the idea that consolidation over time involves the transfer of memories out of the hippocampus to other parts of the brain. However, experimentation has difficulties in testing the sparing of older memories; and, in some cases of retrograde amnesia, the sparing appears to affect memories formed decades before the damage to the hippocampus occurred, so its role in maintaining these older memories remains controversial. Damage to the hippocampus does not affect some aspects of memory, such as the ability to learn new skills (playing a musical instrument, for example), suggesting that such abilities depend on a different type of memory (procedural memory) and different brain regions. And there is evidence (e.g., O'Kane et al 2004) to suggest that patient HM (who had his medial temporal lobes removed bilaterally as a treatment for epilepsy) can form new semantic memories.
forms of memory often last a lifetime, the hippocampus ceases to play a crucial role in the retention of the memory after a period of consolidation. Damage to the hippocampus usually results in profound difficulties in forming new memories (anterograde amnesia), and normally also affects access to memories prior to the damage (retrograde amnesia). Although the retrograde effect normally extends some years prior to the brain damage, in some cases older memories remain - this sparing of older memories leads to the idea that consolidation over time involves the transfer of memories out of the hippocampus to other parts of the brain. However, experimentation has difficulties in testing the sparing of older memories; and, in some cases of retrograde amnesia, the sparing appears to affect memories formed decades before the damage to the hippocampus occurred, so its role in maintaining these older memories remains controversial. Damage to the hippocampus does not affect some aspects of memory, such as the ability to learn new skills (playing a musical instrument, for example), suggesting that such abilities depend on a different type of memory (procedural memory) and different brain regions. And there is evidence (e.g., O'Kane et al 2004) to suggest that patient HM (who had his medial temporal lobes removed bilaterally as a treatment for epilepsy) can form new semantic memories.
 forms of memory often last a lifetime, the hippocampus ceases to play a crucial role in the retention of the memory after a period of consolidation. Damage to the hippocampus usually results in profound difficulties in forming new memories (anterograde amnesia), and normally also affects access to memories prior to the damage (retrograde amnesia). Although the retrograde effect normally extends some years prior to the brain damage, in some cases older memories remain - this sparing of older memories leads to the idea that consolidation over time involves the transfer of memories out of the hippocampus to other parts of the brain. However, experimentation has difficulties in testing the sparing of older memories; and, in some cases of retrograde amnesia, the sparing appears to affect memories formed decades before the damage to the hippocampus occurred, so its role in maintaining these older memories remains controversial. Damage to the hippocampus does not affect some aspects of memory, such as the ability to learn new skills (playing a musical instrument, for example), suggesting that such abilities depend on a different type of memory (procedural memory) and different brain regions. And there is evidence (e.g., O'Kane et al 2004) to suggest that patient HM (who had his medial temporal lobes removed bilaterally as a treatment for epilepsy) can form new semantic memories.
forms of memory often last a lifetime, the hippocampus ceases to play a crucial role in the retention of the memory after a period of consolidation. Damage to the hippocampus usually results in profound difficulties in forming new memories (anterograde amnesia), and normally also affects access to memories prior to the damage (retrograde amnesia). Although the retrograde effect normally extends some years prior to the brain damage, in some cases older memories remain - this sparing of older memories leads to the idea that consolidation over time involves the transfer of memories out of the hippocampus to other parts of the brain. However, experimentation has difficulties in testing the sparing of older memories; and, in some cases of retrograde amnesia, the sparing appears to affect memories formed decades before the damage to the hippocampus occurred, so its role in maintaining these older memories remains controversial. Damage to the hippocampus does not affect some aspects of memory, such as the ability to learn new skills (playing a musical instrument, for example), suggesting that such abilities depend on a different type of memory (procedural memory) and different brain regions. And there is evidence (e.g., O'Kane et al 2004) to suggest that patient HM (who had his medial temporal lobes removed bilaterally as a treatment for epilepsy) can form new semantic memories. Role of Hippocampus in spatial memory and navigation
Some evidence implicates the hippocampus in storing and processing spatial information. Studies in rats have shown that neurons in the hippocampus have spatial firing fields. These cells are called place cells. Some cells fire when the animal finds itself in a particular location, regardless of direction of travel, while most are at least partially sensitive to head direction and direction of travel. In rats, some cells, termed context-dependent cells, may alter their firing depending on the animal's recent past (retrospective) or expected future (prospective). Different cells fire at different locations, so that, by looking at the firing of the cells alone, it becomes possible to tell where the animal is. Place cells have now been seen in humans involved in finding their way around in a virtual reality town. The findings resulted from research with individuals that had electrodes implanted in their brains as a diagnostic part of surgical treatment for serious epilepsy. The discovery of place cells led to the idea that the hippocampus might act as a cognitive map — a neural representation of the layout of the environment. Recent evidence has cast doubt on this perspective, indicating that the hippocampus might be crucial for more fundamental processes within navigation. Regardless, studies with animals have shown that an intact hippocampus is required for simple spatial memory tasks (for instance, finding the way back to a hidden goal). Without a fully-functional hippocampus, humans may not successfully remember where they have been and how to get where they are going. Researchers believe that the hippocampus plays a particularly important role in finding shortcuts and new routes between familiar places. Some people exhibit more skill at this sort of navigation than do others, and brain imaging shows that these individuals have more active hippocampi when navigating. London's taxi drivers must learn a large number of places — and know the most direct routes between them (they have to pass a strict test, The Knowledge, before being licensed to drive the famous black cabs). A study at University College London (Maguire et al, 2000) showed that part of the hippocampus is larger in taxi drivers than in the general public, and that more-experienced drivers have bigger hippocampi. Whether having a bigger hippocampus helps an individual to become a cab driver or finding shortcuts for a living makes an individual's hippocampus grow is yet to be elucidated. A study on rats at Indiana University suggested that the sexual dimorphism in the hippocampus morphology is tied to a sexual dimorphism in repeated maze performance. Males seem to be better at contexualizing their whereabouts because they have more hippocampus to work with.
Now let us know more about cerebral cortex
The cerebral cortex is a brain structure in vertebrates. In non-living, preserved brains, the outermost layers of the cerebrum has a grey color, hence the name "grey matter". Grey matter is formed by neurons and their unmyelinated fibers while the white matter below the grey matter of the cortex is formed predominantly by myelinated axons interconnecting different  regions of the central nervous system. The human cerebral cortex is 2-4 mm (0.08-0.16 inches) thick and plays a central role in many complex brain functions including memory, attention, perceptual awareness, "thinking", language and consciousness. The surface of the cerebral cortex is folded in large mammals like humans, where more than two thirds of the cortical surface is buried in the grooves, called "sulci". The phylogenetically more ancient part of the cerebral cortex, the hippocampus, is differentiated in five layers, while the more recent neo-cortex is differentiated in six basic layers. Relative variations in thickness or cell type (among other parametres) allows us to distinguish among different neocortical architectonic fields. The geometry of these fields seems to be related to the anatomy of the cortical folds and, for example, layers in the upper part of the cortical grooves (called gyri) are more clearly differentiated than in its deeper parts (called sulcal "fundi").
regions of the central nervous system. The human cerebral cortex is 2-4 mm (0.08-0.16 inches) thick and plays a central role in many complex brain functions including memory, attention, perceptual awareness, "thinking", language and consciousness. The surface of the cerebral cortex is folded in large mammals like humans, where more than two thirds of the cortical surface is buried in the grooves, called "sulci". The phylogenetically more ancient part of the cerebral cortex, the hippocampus, is differentiated in five layers, while the more recent neo-cortex is differentiated in six basic layers. Relative variations in thickness or cell type (among other parametres) allows us to distinguish among different neocortical architectonic fields. The geometry of these fields seems to be related to the anatomy of the cortical folds and, for example, layers in the upper part of the cortical grooves (called gyri) are more clearly differentiated than in its deeper parts (called sulcal "fundi").
 regions of the central nervous system. The human cerebral cortex is 2-4 mm (0.08-0.16 inches) thick and plays a central role in many complex brain functions including memory, attention, perceptual awareness, "thinking", language and consciousness. The surface of the cerebral cortex is folded in large mammals like humans, where more than two thirds of the cortical surface is buried in the grooves, called "sulci". The phylogenetically more ancient part of the cerebral cortex, the hippocampus, is differentiated in five layers, while the more recent neo-cortex is differentiated in six basic layers. Relative variations in thickness or cell type (among other parametres) allows us to distinguish among different neocortical architectonic fields. The geometry of these fields seems to be related to the anatomy of the cortical folds and, for example, layers in the upper part of the cortical grooves (called gyri) are more clearly differentiated than in its deeper parts (called sulcal "fundi").
regions of the central nervous system. The human cerebral cortex is 2-4 mm (0.08-0.16 inches) thick and plays a central role in many complex brain functions including memory, attention, perceptual awareness, "thinking", language and consciousness. The surface of the cerebral cortex is folded in large mammals like humans, where more than two thirds of the cortical surface is buried in the grooves, called "sulci". The phylogenetically more ancient part of the cerebral cortex, the hippocampus, is differentiated in five layers, while the more recent neo-cortex is differentiated in six basic layers. Relative variations in thickness or cell type (among other parametres) allows us to distinguish among different neocortical architectonic fields. The geometry of these fields seems to be related to the anatomy of the cortical folds and, for example, layers in the upper part of the cortical grooves (called gyri) are more clearly differentiated than in its deeper parts (called sulcal "fundi"). Now what can go wrong with Memory?
As wonderful as memory is, it isn't always perfect. It's normal to occasionally forget the name of somebody you just met or where you put your shoes[U said Chromosome, may be]. And of course, everyone has forgotten an answer on a test. Darn! You knew that one, too! It's also typical for people to forget more things as they grow older. Your parents or grandparents might joke about having a "senior moment." That's when they forget something. But some memory problems are serious, such as when a person has Alzheimer's disease. Strokes, which also affect older people, are another medical problem that can affect someone's memory. A stroke is when the blood supply to the brain is temporarily stopped or when a blood vessel bursts. Another problem could occuer, that is TBI or Traumatic Brain Injury which can impair your memory for forever.
Wednesday, October 11, 2006
Andrew Fire & Mello shares Nobel Prize for discovering how double-stranded RNA can switch off genes


Andrew Fire took his first look around at Stanford and started screaming. His response wasn't unusual—for a newborn, that is. The molecular biologist was born at Stanford Hospital, attended public schools in Sunnyvale and graduated from the University of California-Berkeley, after being turned down by his only other college choice: Stanford.
All pretty normal, he hastens to point out—not mentioning that he completed high school at age 15 and college at age 19. But as of 2:30 a.m. Monday, the quiet Stanford medical school professor with the self-deprecating air will have to work a little harder to convince the world that he's nothing special. He won this year's Nobel Prize in Physiology or Medicine, and it will be a long time before he sees "normal" again.
Linda Cicero
Andrew Fire
Fire shares the prize with Craig Mello of the University of Massachusetts Medical School. The announcement from the Nobel Assembly at Karolinska Institutet came a mere eight years after they published their breakthrough discovery of RNA interference. The relatively rapid recognition is unusual in the rarified Nobel world, which often rewards researchers decades after their initial findings.
"I was very surprised," said Fire, professor of pathology and of genetics, of the early morning phone call from the committee. "At first I thought that maybe they had a wrong number, or that I was dreaming. But I guess it's real." Such prompt accolades are one indication of how their finding has turned the field of molecular biology on its head—and how it hasn't yet stopped spinning.
"This is an extraordinary achievement for Andy Fire and Craig Mello, for science and for Stanford," said Philip Pizzo, MD, dean of the School of Medicine. "It affirms the importance of basic fundamental research, which often yields new insights into human biology. Their discovery is already unfolding in new directions that may translate into discoveries of new diagnostic and therapeutic approaches for a variety of human disorders."
Fire, PhD, 47, and Mello, PhD, 45, are part of a team of researchers credited with recognizing that certain RNA molecules can be used to turn off specific genes in animal cells. The discovery, made while Fire was at the Carnegie Institution's Department of Embryology in Baltimore, marked the first time that biologists were able to selectively "silence" the voice of one gene in the cacophony of the tens of thousands that give a cell its marching orders from development to death. Their description of the process, called RNA interference or RNAi, in Nature in 1998, jumpstarted a new biological field by opening up previously inaccessible areas of research.
"It was clear from the first week that I met Andy that he was destined to do something great," said a longtime friend and Carnegie Institution colleague David Schwartz, PhD, professor of genetics and of chemistry at the University of Wisconsin-Madison. "He was just such a natural about it. There are people who are excellent at sports, you just put a baseball bat in their hands and the ball flies. Andy is like that with science; without a fuss, it just happens."
Before the discovery, the only method of removing a gene's influence from a population of cells involved a laborious and time-consuming series of experiments with no guarantee of success. It was virtually impossible to "knock-out" even a small fraction of genetic suspects in a particular pathway. Now researchers around the world are using RNAi techniques to quickly and randomly silence one gene at a time in swaths of cells. By plucking out those that act abnormally with regard to the pathway in question, they are able to identify even previously unknown genes involved in the pathway.
Linda Cicero
Andrew Fire (right), with Stanford President John Hennessy, became the third member of the medical school faculty to win a Nobel Prize.
The technique has also shown remarkable clinical promise. RNAi-based treatments are being tested in many animal models of disease—high cholesterol, HIV, cancer and hepatitis, among others—and clinical trials have been launched in humans with specific types of macular degeneration and pneumonia. The potential applications of the research are vast.
Despite some intriguing hints that RNA was more than just an assembly manual for proteins, much of this process remained a mystery until Fire and Mello published their findings in the nematode C. elegans, a tiny worm about the width of a No. 2 pencil lead. But Fire emphasizes that much of the preliminary legwork had already been done by other plant and animal researchers.
"We came into a field where a lot was already known," said Fire. "It was a complex jigsaw puzzle, and we were able to contribute one piece. Fortunately for us it was a very nice piece, but it would be really disingenuous to say we did the whole puzzle."
Such demurring is standard for Fire; colleagues often describe him as remarkably modest. Monday, Fire lived up to that reputation. After reluctantly agreeing to participate in numerous media interviews and press conferences, he made sure to credit "insightful and dedicated colleagues and students" with whom he has worked and "whose ideas and efforts are very much the subject of the prize." And he noted that scientists have a responsibility to society at large. "All of us in science look forward to sharing with the public both the responsibilities and opportunities that arise as we understand more about the human body," he said.
Fire added, "For me personally, the occasion of such an award is an opportunity to thank the many patient teachers and mentors who have opened doors to science and research, and especially my family, who have made everything possible.
"This day is a wonderful chance to acknowledge that science is a group effort," Fire continued. "The advances cited in the Nobel award grew from original scientific inquiry from numerous research groups throughout the world." He also thanked the National Institute of General Medical Sciences for providing the grants that made the research possible and continues to support both scientists.
Others were just a little more effervescent. "My wife and I have known him for 20 years, and we were jumping and hooting and hollering when we found out," said Schwartz, who is also the director of the Genomic Sciences Training Program at Madison's Laboratory for Molecular and Computational Genomics. "I spoke to him a couple of weeks ago and told him he was going to win the prize. With his typical understated personality, he said 'Let's talk about something else.'" In fact, Fire is so unassuming that he first suspected his early morning phone call was a prank by his old friend.
Fire will officially receive the award on Dec. 10 in Stockholm, and he and Mello will share the $1.4 million prize. He is the medical school's third Nobel laureate, joining emeritus professors Paul Berg, PhD, and Arthur Kornberg, MD.
"Professor Fire's contributions to his field have been of enormous importance and the recognition by the Nobel committee is a remarkable achievement at this early point in his career," said President John Hennessy. "The RNA research of professors Fire and Mello represents the very best of the collaborative nature of university scholarship. The fact that this basic discovery is already impacting the development of new therapies is a wonderful reminder of the importance of fundamental research."
As any graduate student can attest, fundamental research often means long hours of tedium. Although Fire is careful to credit others, he's no stranger to such drudgery. "I'd be working in the middle of the night," recalled Schwartz, "and Andy would be hunched over his microscope next door, feeding his worms. They had a mutation that made them so uncoordinated that he had to push food their way with a tiny brush." But the work paid off. "This is just gorgeous work that stands a chance to really change medicine, as well as being a remarkable tool for biology," said Schwartz. "Anyone who knows him will not be surprised that he won."
After Fire received his PhD from MIT, he was accepted as a Helen Hay Whitney Postdoctoral Fellow in Cambridge, England, in a laboratory headed by Nobel laureate Sydney Brenner, PhD. He conducted his initial work on gene silencing by double-stranded RNA between 1986 and 2003 while at the Carnegie Institution. He was an adjunct professor in the Department of Biology at Johns Hopkins University starting in 1989 and joined the Stanford faculty in 2003. Throughout his career, all of the major work in Fire's lab has been supported by research grants from the US National Institutes of Health.
Fire is a member of the National Academy of Sciences and of the American Academy of Arts & Sciences. He serves on the Board of Scientific Counselors and the NIH's National Center for Biotechnology. He has received and shared numerous awards, including the Maryland Distinguished Young Scientist Award, Meyenburg Prize, Genetics Society of America Medal, National Academy of Sciences Award in Molecular Biology, Passano Family Foundation Award, Wiley Prize, H.P. Heineken Prize in Biochemistry and Biophysics, Warren Triennial Prize, Rosenstiel Award, Gairdner Award, Massry Prize and Ehrlich/Darmstaedter Prize.
None of this has gone to Fire's head—and the Nobel Prize doesn't appear to be either. "I like what I do," he said when asked how the Nobel might affect his life. "I like teaching, I like research and I like talking to colleagues. This brings another dimension: an opportunity to have a voice beyond my own lab and field. That's a big responsibility, and I look forward to using that voice as needed. At the same time, I still want to do interesting and unusual experiments, while also making sure I don't get too much credit."
All pretty normal, he hastens to point out—not mentioning that he completed high school at age 15 and college at age 19. But as of 2:30 a.m. Monday, the quiet Stanford medical school professor with the self-deprecating air will have to work a little harder to convince the world that he's nothing special. He won this year's Nobel Prize in Physiology or Medicine, and it will be a long time before he sees "normal" again.
Linda Cicero
Andrew Fire
Fire shares the prize with Craig Mello of the University of Massachusetts Medical School. The announcement from the Nobel Assembly at Karolinska Institutet came a mere eight years after they published their breakthrough discovery of RNA interference. The relatively rapid recognition is unusual in the rarified Nobel world, which often rewards researchers decades after their initial findings.
"I was very surprised," said Fire, professor of pathology and of genetics, of the early morning phone call from the committee. "At first I thought that maybe they had a wrong number, or that I was dreaming. But I guess it's real." Such prompt accolades are one indication of how their finding has turned the field of molecular biology on its head—and how it hasn't yet stopped spinning.
"This is an extraordinary achievement for Andy Fire and Craig Mello, for science and for Stanford," said Philip Pizzo, MD, dean of the School of Medicine. "It affirms the importance of basic fundamental research, which often yields new insights into human biology. Their discovery is already unfolding in new directions that may translate into discoveries of new diagnostic and therapeutic approaches for a variety of human disorders."
Fire, PhD, 47, and Mello, PhD, 45, are part of a team of researchers credited with recognizing that certain RNA molecules can be used to turn off specific genes in animal cells. The discovery, made while Fire was at the Carnegie Institution's Department of Embryology in Baltimore, marked the first time that biologists were able to selectively "silence" the voice of one gene in the cacophony of the tens of thousands that give a cell its marching orders from development to death. Their description of the process, called RNA interference or RNAi, in Nature in 1998, jumpstarted a new biological field by opening up previously inaccessible areas of research.
"It was clear from the first week that I met Andy that he was destined to do something great," said a longtime friend and Carnegie Institution colleague David Schwartz, PhD, professor of genetics and of chemistry at the University of Wisconsin-Madison. "He was just such a natural about it. There are people who are excellent at sports, you just put a baseball bat in their hands and the ball flies. Andy is like that with science; without a fuss, it just happens."
Before the discovery, the only method of removing a gene's influence from a population of cells involved a laborious and time-consuming series of experiments with no guarantee of success. It was virtually impossible to "knock-out" even a small fraction of genetic suspects in a particular pathway. Now researchers around the world are using RNAi techniques to quickly and randomly silence one gene at a time in swaths of cells. By plucking out those that act abnormally with regard to the pathway in question, they are able to identify even previously unknown genes involved in the pathway.
Linda Cicero
Andrew Fire (right), with Stanford President John Hennessy, became the third member of the medical school faculty to win a Nobel Prize.
The technique has also shown remarkable clinical promise. RNAi-based treatments are being tested in many animal models of disease—high cholesterol, HIV, cancer and hepatitis, among others—and clinical trials have been launched in humans with specific types of macular degeneration and pneumonia. The potential applications of the research are vast.
Despite some intriguing hints that RNA was more than just an assembly manual for proteins, much of this process remained a mystery until Fire and Mello published their findings in the nematode C. elegans, a tiny worm about the width of a No. 2 pencil lead. But Fire emphasizes that much of the preliminary legwork had already been done by other plant and animal researchers.
"We came into a field where a lot was already known," said Fire. "It was a complex jigsaw puzzle, and we were able to contribute one piece. Fortunately for us it was a very nice piece, but it would be really disingenuous to say we did the whole puzzle."
Such demurring is standard for Fire; colleagues often describe him as remarkably modest. Monday, Fire lived up to that reputation. After reluctantly agreeing to participate in numerous media interviews and press conferences, he made sure to credit "insightful and dedicated colleagues and students" with whom he has worked and "whose ideas and efforts are very much the subject of the prize." And he noted that scientists have a responsibility to society at large. "All of us in science look forward to sharing with the public both the responsibilities and opportunities that arise as we understand more about the human body," he said.
Fire added, "For me personally, the occasion of such an award is an opportunity to thank the many patient teachers and mentors who have opened doors to science and research, and especially my family, who have made everything possible.
"This day is a wonderful chance to acknowledge that science is a group effort," Fire continued. "The advances cited in the Nobel award grew from original scientific inquiry from numerous research groups throughout the world." He also thanked the National Institute of General Medical Sciences for providing the grants that made the research possible and continues to support both scientists.
Others were just a little more effervescent. "My wife and I have known him for 20 years, and we were jumping and hooting and hollering when we found out," said Schwartz, who is also the director of the Genomic Sciences Training Program at Madison's Laboratory for Molecular and Computational Genomics. "I spoke to him a couple of weeks ago and told him he was going to win the prize. With his typical understated personality, he said 'Let's talk about something else.'" In fact, Fire is so unassuming that he first suspected his early morning phone call was a prank by his old friend.
Fire will officially receive the award on Dec. 10 in Stockholm, and he and Mello will share the $1.4 million prize. He is the medical school's third Nobel laureate, joining emeritus professors Paul Berg, PhD, and Arthur Kornberg, MD.
"Professor Fire's contributions to his field have been of enormous importance and the recognition by the Nobel committee is a remarkable achievement at this early point in his career," said President John Hennessy. "The RNA research of professors Fire and Mello represents the very best of the collaborative nature of university scholarship. The fact that this basic discovery is already impacting the development of new therapies is a wonderful reminder of the importance of fundamental research."
As any graduate student can attest, fundamental research often means long hours of tedium. Although Fire is careful to credit others, he's no stranger to such drudgery. "I'd be working in the middle of the night," recalled Schwartz, "and Andy would be hunched over his microscope next door, feeding his worms. They had a mutation that made them so uncoordinated that he had to push food their way with a tiny brush." But the work paid off. "This is just gorgeous work that stands a chance to really change medicine, as well as being a remarkable tool for biology," said Schwartz. "Anyone who knows him will not be surprised that he won."
After Fire received his PhD from MIT, he was accepted as a Helen Hay Whitney Postdoctoral Fellow in Cambridge, England, in a laboratory headed by Nobel laureate Sydney Brenner, PhD. He conducted his initial work on gene silencing by double-stranded RNA between 1986 and 2003 while at the Carnegie Institution. He was an adjunct professor in the Department of Biology at Johns Hopkins University starting in 1989 and joined the Stanford faculty in 2003. Throughout his career, all of the major work in Fire's lab has been supported by research grants from the US National Institutes of Health.
Fire is a member of the National Academy of Sciences and of the American Academy of Arts & Sciences. He serves on the Board of Scientific Counselors and the NIH's National Center for Biotechnology. He has received and shared numerous awards, including the Maryland Distinguished Young Scientist Award, Meyenburg Prize, Genetics Society of America Medal, National Academy of Sciences Award in Molecular Biology, Passano Family Foundation Award, Wiley Prize, H.P. Heineken Prize in Biochemistry and Biophysics, Warren Triennial Prize, Rosenstiel Award, Gairdner Award, Massry Prize and Ehrlich/Darmstaedter Prize.
None of this has gone to Fire's head—and the Nobel Prize doesn't appear to be either. "I like what I do," he said when asked how the Nobel might affect his life. "I like teaching, I like research and I like talking to colleagues. This brings another dimension: an opportunity to have a voice beyond my own lab and field. That's a big responsibility, and I look forward to using that voice as needed. At the same time, I still want to do interesting and unusual experiments, while also making sure I don't get too much credit."
By KRISTA CONGER[STANFORD UNIVERSITY]
Mutation in (mt)DNA causes Human diseases
A number of human diseases are caused by mutations in genes in our mitochondria: cytochrome b 12S rRNA ATP synthase subunits of NADH dehydrogenase several tRNA genes Although many different organs may be affected, disorders of the brain and muscles are the most common. Perhaps this reflects the great demand for energy of both these organs. Some of these disorders are inherited in the germline. In every case, the mutant gene is received from the mother because none of the mitochondria in sperm survives in the fertilized egg. Other disorders are somatic; that is, the mutation occurs in the somatic tissues of the individual. Example: exercise intolerance A number of humans who suffer from easily-fatigued muscles turn out to have a mutations in their cytochrome b gene. Curiously, only the mitochondria in their muscles have the mutation; the mtDNA of their other tissues is normal. Presumably, very early in their embryonic development, a mutation occurred in a cytochrome b gene in the mitochondrion of a cell destined to produce their muscles. The severity of mitochondrial diseases varies greatly. The reason for this is probably the extensive mixing of mutant DNA and normal DNA in the mitochondria as they fuse with one another. A mixture of both is called heteroplasmy. The higher the ratio of mutant to normal, the greater the severity of the disease. In fact by chance alone, cells can on occasion end up with all their mitochondria carrying all-mutant genomes — a condition called homoplasmy (a phenomenon resembling genetic drift).
DIP JYOTI CHAKRABORTY
DIP JYOTI CHAKRABORTY
Sunday, October 08, 2006
SCIENTIST DISCOVER NEW EPILEPSY GENE
It is a boon to the genetical students and epileptic patients that Harvard Medical School found a new gene causes epilepsy.
Here the report goes, "Boston--Harvard Medical School affiliate Beth Israel Deaconess Medical Center--Scientists studying the genetic basis of epilepsy have discovered a gene that is not only required for proper brain development, but which also may play an important role in vascular disease and stroke. Researchers at Boston's Beth Israel Deaconess Medical Center for the first time have found that the gene filamin 1 (Flnl), which has previously been implicated in platelet function and cell motility outside the central nervous system, plays a critical role in human brain development and causes epilepsy when its normal function is disrupted. They also report that patients with F1n1 mutations are prone to suffering strokes and often are born with a serious vascular anomaly called patent ductus arteriosus. Their findings are the cover story of the December Neuron. "No one realized until now that this gene played a role in the brain" says Christopher A. Walsh, MD, PhD, director of the neurogenetics laboratory at Beth Israel Deaconess Medical Center, and principal investigator of the Neuron study, "Mutations in Filamin 1 Prevent Migration of Cerebral Cortical Neurons in Human Periventricular Heterotopia." "We are, of course excited about the role this gene is playing in the developing brain, but we are also interested in F1n1's connections to stroke and vascular development," continues Walsh. Periventricular heterotopia (PH) is a disorder in which neurons in the brain fail to migrate to their proper location during development, and which results in epilepsy in affected females. Mutations in F1n1 cause this disorder in females, while males with F1n1 mutations die before birth, presumably due to an essential role F1n1 has in the development of the embryo. Walsh and colleagues first homed in on the gene for PH by studying families in which the disorder is inherited. Using gene mapping techniques they narrowed down their search from a possible 60,000 genes to approximately 100 genes. They then studied about a dozen of these genes to determine if any were mutated in their PH patients. Walsh estimates that he and his team spent about six person-years mapping and testing genes before mutations in F1n1 were identified. This gene-hunt has been a full time job for the past three and a half years for the first author of the Neuron paper, Jeremy Fox. "This kind of work is very unpredictable. What pays off in the end is persistence," comments Fox. Walsh speculates that F1n1 acts as an engine that initiates cell migration from deep within the brain to the area where development of the cortex takes place. With some of this migration blocked by F1n1 mutations, many neurons remain deep within the brain and possibly cause epilepsy by making inappropriate connections. Patients with PH mutations also often suffer from strokes and vasculature defects, and Walsh believes that more subtle F1n1 mutations may be found in many people with these other problems. His group plans to collaborate with colleagues at Harvard Medical School to study the non-neuronal roles of F1n1, while continuing studies into the precise role of F1n1 in migrating neurons in the developing brain."
DIP JYOTI CHAKRABORTY
Here the report goes, "Boston--Harvard Medical School affiliate Beth Israel Deaconess Medical Center--Scientists studying the genetic basis of epilepsy have discovered a gene that is not only required for proper brain development, but which also may play an important role in vascular disease and stroke. Researchers at Boston's Beth Israel Deaconess Medical Center for the first time have found that the gene filamin 1 (Flnl), which has previously been implicated in platelet function and cell motility outside the central nervous system, plays a critical role in human brain development and causes epilepsy when its normal function is disrupted. They also report that patients with F1n1 mutations are prone to suffering strokes and often are born with a serious vascular anomaly called patent ductus arteriosus. Their findings are the cover story of the December Neuron. "No one realized until now that this gene played a role in the brain" says Christopher A. Walsh, MD, PhD, director of the neurogenetics laboratory at Beth Israel Deaconess Medical Center, and principal investigator of the Neuron study, "Mutations in Filamin 1 Prevent Migration of Cerebral Cortical Neurons in Human Periventricular Heterotopia." "We are, of course excited about the role this gene is playing in the developing brain, but we are also interested in F1n1's connections to stroke and vascular development," continues Walsh. Periventricular heterotopia (PH) is a disorder in which neurons in the brain fail to migrate to their proper location during development, and which results in epilepsy in affected females. Mutations in F1n1 cause this disorder in females, while males with F1n1 mutations die before birth, presumably due to an essential role F1n1 has in the development of the embryo. Walsh and colleagues first homed in on the gene for PH by studying families in which the disorder is inherited. Using gene mapping techniques they narrowed down their search from a possible 60,000 genes to approximately 100 genes. They then studied about a dozen of these genes to determine if any were mutated in their PH patients. Walsh estimates that he and his team spent about six person-years mapping and testing genes before mutations in F1n1 were identified. This gene-hunt has been a full time job for the past three and a half years for the first author of the Neuron paper, Jeremy Fox. "This kind of work is very unpredictable. What pays off in the end is persistence," comments Fox. Walsh speculates that F1n1 acts as an engine that initiates cell migration from deep within the brain to the area where development of the cortex takes place. With some of this migration blocked by F1n1 mutations, many neurons remain deep within the brain and possibly cause epilepsy by making inappropriate connections. Patients with PH mutations also often suffer from strokes and vasculature defects, and Walsh believes that more subtle F1n1 mutations may be found in many people with these other problems. His group plans to collaborate with colleagues at Harvard Medical School to study the non-neuronal roles of F1n1, while continuing studies into the precise role of F1n1 in migrating neurons in the developing brain."
DIP JYOTI CHAKRABORTY
Subscribe to:
Comments (Atom)


